
Aggregating patients for discharge readiness in the neonatal intensive care unit: impact on length of stay
Highlight box
Key findings
• This study highlights a new team structure model to aid in more efficient length of stay (LOS) in the neonatal intensive care unit (NICU).
What is known and what is new?
• Studies from the emergency department have shown decreased LOS when patients are aggregated by acuity level, however, this is lacking in the field of neonatology.
• This study adds new team structures to promote more efficient LOS in the NICU.
What is the implication, and what should change now?
• This study provides a team design to optimize LOS for infants with efficient discharge planning, ultimately leading to decreased health care cost and resource utilization.
Introduction
In 2021, 1 in 10 infants in the United States were born preterm [less than 37 weeks’ gestational age (GA)] (1). A retrospective study of infants in California from 2009–2011 reported premature infants incurred 61% of all newborn hospital costs (2). The estimated cost of preterm infants in economic terms, including medical, educational, and lost productivity, amounted to over $25 billion in 2016 (3). The mean hospital-related cost for a full-term infant is $2,433 with an average length of stay (LOS) of about 3 days. The average LOS for an infant less than 37 weeks’ gestation was 15 days and a mean cost of $48,036. Compared to an infant born less than 32 weeks’ gestation who had an average LOS of 58 days and cost of $223,931 (2).
In the past 30 years, neonatal intensive care unit (NICU) layout in the United States has steadily shifted from open bay wards to single family room models due to an association between single family room NICUs and a decrease in both LOS and duration of ventilator support (4). However, there is no consensus on a regulated national standard for the layout and flow of a NICU. Considering that lower acuity infants may be negatively impacted in open bay ward NICUs due to increased exposure to noise and potential stressful interruptions in sleep and growth likely contributing to increased LOS, NICU design may be more impactful than previously understood. Historically, open bay NICUs might have had one bay dedicated as a step-down unit for convalescing infants for discharge readiness. The concept of acuity-based team assignments in single room NICUs with the goal of optimizing LOS has not been previously reported in the literature.
Noise pollution is a factor in infant development that is significantly impacted by the design and layout of a unit. The American Academy of Pediatrics recommends the threshold for continuous noise levels in a NICU should not exceed the 45-decibel range (5). Evidence provided by a French academic medical center showed that the sound levels in sampled NICUs had exceeded that threshold, particularly within an incubator, potentially negatively impacting neonatal sensory development (6). Infants’ high acuity status is accentuated with the requirement of additional equipment such as ventilators and multiple medication pumps. Clustering infants by acuity level might lead to infants with lower acuity status achieving more restful states to feed and grow when physically moved away from higher acuity areas.
An emergency department (ED) model where patients are aggregated by acuity level has shown a decrease in door-to-discharge time for five of the top 12 most common complaints within EDs, including a 5.4% reduction for all patients (7). Per review of available literature, no prior studies have examined acuity level aggregation for infants in the NICU. We hypothesized that aggregating patients by acuity level for discharge readiness in the NICU would be associated with shorter LOS. We present this article in accordance with the STROBE reporting checklist (available at https://jhmhp.amegroups.com/article/view/10.21037/jhmhp-23-71/rc).
Methods
Study design
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Rush University Institutional Review Board (ORA No. 20102202-IRB01) and individual consent for this retrospective analysis was waived.
This retrospective, pre-post study evaluating LOS included 1,118 infants who were admitted and discharged from the NICU between July 1, 2016 and December 31, 2019. The NICU is a level III 60-bed, single family room, unit within a large academic medical center in an urban setting, located in the Midwest region of the United States. Infants were excluded from the study if they had a NICU stay less than 5 days, died before discharge, were transferred to another facility, or received extracorporeal membrane oxygenation (ECMO) or tracheostomy. Additionally, patients were excluded if there was any missing information for key variables. Data were collected from the organization’s electronic data warehouse, a repository for electronic medical record data, and the organization’s financial data mart.
NICU team structure
Prior to this study’s intervention, patients in the NICU were assigned a care team without regard to acuity level, and the team assignment did not change during the NICU stay. In April 2018 the NICU implemented an acuity-based model for assigning patients to care teams for the duration of their NICU stay. A three-team system was created with two higher acuity level teams and one lower acuity level team. This model of clustering infants by their acuity level was implemented to ensure lower acuity infants received dedicated preparation for discharge readiness. In general, patients on the lower acuity team were geographically in close proximity to each other in the NICU. This new team structure dynamic mimics staffing models in hospitals with a dedicated step-down unit in a separate area of the hospital, with specific staff dedicated to lower acuity infants and discharge preparation. The goal of the new team structure was to minimize time and attention likely taken away from lower acuity infants when intermixed on the same team with higher acuity infants to eliminate workflow-related delays in the discharge preparation process.
Each infant who was admitted to the NICU was individually evaluated by the care team who reviewed the infant’s GA by week, birth weight, morbidities, and clinical factors, and the NICU’s current staffing when determining the care team assignment for the patient during the stay. Two teams were characterized by their mix of high-acuity patients, while a third team was primarily for lower-acuity patients, such as convalescing premature infants and those preparing for discharge. The majority of infants from the higher acuity teams transition to the lower acuity team when their acuity was reduced in preparation for discharge, such as a very low birth weight infant or very preterm infant would transition to the lower acuity team when advanced to feeder—grower status.
Definition of measures
The dependent variable of interest in the study was infant NICU LOS. The primary independent variable of interest was time period, indicating whether the infant was admitted prior to aggregating patients by acuity level for discharge readiness (pre-aggregation period, July 1, 2016–March 31, 2018) or after implementation of the aggregation design (post-aggregation period, April 1, 2018–December 31, 2019). Other covariates included infant and maternal characteristics.
Infant characteristics were demographic characteristics, clinical factors and acuity. Infant demographic characteristics included sex and race/ethnicity (non-Hispanic Black, Hispanic, non-Hispanic White, and other racial/ethnic groups). Infant clinical factors included infant GA either a continuous variable in weeks or categorical variable (<28, 28–31, 32–33, 34–36, 37 weeks and older), and small for GA at birth, defined as less than the 10th percentile of weight for GA (8). Acuity was measured by GA <33 weeks, as those infants typically require respiratory support at birth ranging from continuous positive airway pressure to intubation; other high acuity diagnosis at birth (e.g., respiratory failure of newborn, hypotension of newborn, pneumothorax); presence of any complication of prematurity that occurred during the NICU hospitalization [bronchopulmonary dysplasia, necrotizing enterocolitis, retinopathy of prematurity or intraventricular hemorrhage, based on International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10) diagnosis codes]; and diagnosis of patent ductus arteriosus (PDA), which can cause hemodynamic instability. Infant acuity at birth was measured using a set of inclusion and exclusion guidelines determined by the neonatologists on clinical service using diagnoses present at or near the time of birth and based on clinical judgement. This list of included diagnoses for high acuity was selected by a group of neonatologists who reviewed ICD-10 diagnosis codes available for neonatology, which were limited to those codes that were in these data for the study period (Table S1).
Maternal characteristics included age at time of delivery (years), primary payer (Medicaid and commercial insurance), gravida (1, 2–4, 5 or more), average family visits per week, and median neighborhood household income. The number of family visits per week was collected through the electronic medical record patient’s flowsheet, and the average number of family visits per week was calculated based on visitation during the entire NICU stay. Average weekly family visits were categorized as 2 or fewer, >2–4, >4–6, >6. Median neighborhood household income was based on zip code of home residence using data from the United States Census Bureau.
Statistical analysis
Variables were described using median [interquartile range (IQR)] and frequency distributions, depending on the type of variable. Differences in independent variables were compared between time periods using Mann-Whitney U tests and chi-square tests. Generalized linear regression was used with a negative binomial distribution and log link function to test whether NICU LOS differed between time periods. The first model was an unadjusted model, including only time period as an independent variable. The second model adjusted for infant and maternal characteristics. Average marginal effects for time period were computed by first calculating the predicted NICU LOS (predicted margin) for all infants in the sample as if they were hospitalized in the pre-aggregation period, then calculating the predicted NICU LOS for all infants as if they were hospitalized in the post-aggregation period. The average marginal effect was the difference between the predicted NICU LOS in the two time periods. The 95% confidence intervals (CIs) were calculated for the predicted margin and average marginal effects. Additionally, similar regression models were constructed, stratifying by GA (<28, 28–31, 32–33, 34–36, 37 weeks and older), using the same procedures. Statistical analyses were performed using SAS version 9.4 (Cary, NC, USA) and Stata version 17.0 (College Station, TX, USA).
Results
The study included 1,118 infants who were admitted to and discharged from the NICU between July 1, 2016 and December 31, 2019. Overall, the median GA was 34 weeks (IQR, 31–37 weeks), 43.5% non-Hispanic Black and 29.3% Hispanic, and 63.8% were insured by Medicaid (Table 1). There were no differences in infant or maternal demographic characteristics, family visitation, or infant severity of illness, as measured by high acuity diagnosis at birth, any complications or PDA (Table 1).
Table 1
| Characteristics | Total (n=1,118) | Pre-aggregation (n=543) | Post-aggregation (n=575) | P value |
|---|---|---|---|---|
| Infant characteristics | ||||
| Male sex | 628 (56.2) | 302 (55.6) | 326 (56.7) | 0.716 |
| Race/ethnicity | 0.420 | |||
| Non-Hispanic Black | 486 (43.5) | 241 (44.4) | 245 (42.6) | |
| Hispanic | 327 (29.3) | 149 (27.4) | 178 (31.0) | |
| Non-Hispanic White | 206 (18.4) | 99 (18.2) | 107 (18.6) | |
| Other | 99 (8.9) | 54 (9.9) | 45 (7.8) | |
| GA (weeks) | 34 [31, 37] | 34 [31, 38] | 34 [31.5, 37.0] | 0.852 |
| GA category | 0.148 | |||
| <28 weeks | 120 (10.7) | 62 (11.4) | 58 (10.1) | |
| 28–31 weeks | 181 (16.2) | 95 (17.5) | 86 (15.0) | |
| 32–33 weeks | 177 (15.8) | 81 (14.9) | 96 (16.7) | |
| 34–36 weeks | 288 (25.8) | 124 (22.8) | 164 (28.5) | |
| 37 weeks and older | 352 (31.5) | 181 (33.3) | 171 (29.7) | |
| Small for GA at birth | 180 (16.1) | 93 (17.1) | 87 (15.1) | 0.364 |
| High acuity diagnosis at birth | 979 (87.6) | 468 (86.2) | 511 (88.9) | 0.174 |
| Any complication | 166 (14.9) | 83 (15.3) | 83 (14.4) | 0.689 |
| PDA | 202 (18.1) | 99 (18.2) | 103 (17.9) | 0.890 |
| Maternal characteristics | ||||
| Maternal age (years) | 30 [25, 35] | 30 [25, 34] | 30 [26, 35] | 0.220 |
| Primary payer | 0.247 | |||
| Medicaid | 713 (63.8) | 337 (62.1) | 376 (65.4) | |
| Commercial insurance | 405 (36.2) | 206 (37.9) | 199 (34.6) | |
| Gravida | 0.223 | |||
| 1 | 344 (30.8) | 167 (30.8) | 177 (30.8) | |
| 2–4 | 586 (52.4) | 295 (54.3) | 291 (50.6) | |
| 5 or more | 188 (16.8) | 81 (14.9) | 107 (18.6) | |
| Family visitation, average number per week | 0.442 | |||
| 0–2 | 79 (7.1) | 45 (8.3) | 34 (5.9) | |
| >2–4 | 228 (20.4) | 108 (19.9) | 120 (20.9) | |
| >4–6 | 473 (2.3) | 231 (42.5) | 242 (42.1) | |
| >6 | 338 (30.2) | 159 (29.3) | 179 (31.1) | |
| Median neighborhood income | 0.159 | |||
| <$30k | 95 (8.5) | 47 (8.7) | 48 (8.4) | |
| $30k–<$50k | 407 (36.4) | 185 (34.1) | 222 (38.6) | |
| $50k–<$70k | 305 (27.3) | 145 (26.7) | 160 (27.8) | |
| $70k–<$90k | 157 (14.0) | 90 (16.6) | 67 (11.7) | |
| $90k+ | 154 (13.8) | 76 (14.0) | 78 (13.6) | |
Data are presented as n (%) or median [IQR]. GA, gestational age; PDA, patent ductus arteriosus; IQR, interquartile range.
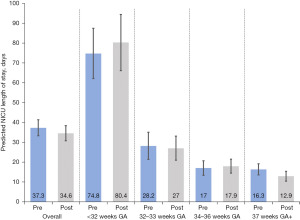
The average unadjusted NICU LOS for infants in the pre-aggregation period was 35.7 days (95% CI, 32.7, 38.7) compared to 33.1 days for infants in the post-aggregation period (95% CI, 30.4, 35.8), and the difference was not statistically significant (Table 2). After adjusting for infant and maternal demographic characteristics, the average marginal effect for the post-aggregation period was −2.7 fewer NICU days (95% CI, −7.2, 1.7) (Figure 1, Table 2; description of sample of infants with GA at birth 37 weeks and older results provided in Table S2). There was no significant different in LOS between aggregation periods for infants born <37 weeks GA, however, LOS significantly decreased for infants born 37 weeks GA or older in the post-aggregation period, with −3.5 fewer NICU days (95% CI, −6.8, −0.1).
Table 2
| Gestational age | N | Predicted NICU LOS (95% CI) (days) | Average marginal effect (difference) (95% CI) | |
|---|---|---|---|---|
| Pre-aggregation | Post-aggregation | |||
| All infants | 1,118 | |||
| Unadjusted model | 35.7 (32.7, 38.7) | 33.1 (30.4, 35.8) | −2.6 (−6.7, 1.5) | |
| Adjusted model† | 37.3 (33.3, 41.4) | 34.6 (30.8, 38.4) | −2.7 (−7.2, 1.7) | |
| <32 weeks GA | 301 | |||
| Unadjusted model | 73.9 (62.3, 85.6) | 81.2 (67.8, 94.5) | 7.2 (−10.5, 24.9) | |
| Adjusted model† | 74.8 (62.1, 87.5) | 80.4 (66.2, 94.5) | 5.6 (−12.9, 24.0) | |
| 32–33 weeks GA | 177 | |||
| Unadjusted model | 28.5 (22.3, 34.8) | 26.4 (21.0, 31.8) | −2.1 (−10.4, 6.1) | |
| Adjusted model† | 28.2 (21.3, 35.1) | 27.0 (20.9, 33.1) | −1.2 (−10.6, 8.2) | |
| 34–36 weeks GA | 288 | |||
| Unadjusted model | 18.1 (14.8, 21.3) | 17.3 (14.6, 20.1) | −0.7 (−5.0, 3.5) | |
| Adjusted model† | 17.0 (13.3, 20.6) | 17.9 (14.4, 21.4) | −0.9 (−3.5, 5.4) | |
| 37 weeks GA and older | 352 | |||
| Unadjusted model | 18.0 (15.3, 20.6) | 11.6 (9.8, 13.5) | −6.3 (−9.6, −3.1) | |
| Adjusted model† | 16.3 (13.5, 19.1) | 12.9 (10.4, 15.3) | −3.5 (−6.8, −0.1) | |
†, adjusted model includes the following covariates: infant characteristics (sex, race/ethnicity, GA in weeks, small for GA at birth, high acuity diagnosis at birth, any complication, PDA, and GA <33 weeks), maternal characteristics (age, primary payer, gravida, average number of family visits per week, median neighborhood household income). NICU, neonatal intensive care unit; LOS, length of stay; CI, confidence interval; GA, gestational age; PDA, patent ductus arteriosus.
Discussion
The purpose of this study was to evaluate the association of grouping patients by acuity level for discharge readiness in the NICU while controlling for additional variables. These variables included infant: sex, race/ethnicity, GA, small for GA, high acuity diagnosis at birth, complications, PDA; and maternal: age, primary insurance payer, gravida, median neighborhood household income, and average family visits per week. We found that all independent variables controlled for, with the exclusion of median household income, had a significant association with LOS. The importance here is not held within the geographic space of clustering each team together but in the formation of care teams that are dedicated to each acuity level that can tailor the level of care required by the patients. The way in which this reduction in LOS was achieved might have been due to several factors including optimizing time infants transition to oral feedings, weaning from respiratory support, and enhancing discharge planning with families.
Infants born 37 weeks GA or older had a significantly shorter LOS in the post-aggregation period compared to infant in the pre-aggregation period, translating to an average reduction of 3.5 days per infant, after adjusting for infant and maternal characteristics. This reduction in LOS also translates into more time for the infant to bond with guardian(s) in a home environment, minimize hospital-based environmental exposures, and likely minimize additional stress and financial impact for guardians maintaining employment and/or caring for additional family members while juggling visiting infants the in NICU (9-12).
This reduction in average LOS spread over hundreds of patients represents a significant amount of cost savings. These cost savings would be the result of a reduction in labor hours, supplies, and the decreased utilization of resources. Hospitals are challenged by the effects of the great resignation as labor expenses are increasing at a higher trend than normal. Additionally, staffing vacancies have required the use of agency/travelers and retention programs which come at a premium. While no one knows if the current trends are here to stay, the ability to manage LOS is helpful in balancing the challenges in the labor market.
Team configurations based on acuity level enable the lower acuity team to prioritize discharge planning and enhance family awareness and participation of infant’s discharge preparation needs. This team structure could potentially be generalizable to other units based on staffing models and team design. A separate physical geographical area for the team is not necessary, as our model demonstrated the effect with team coverage delineation.
There were several limitations to this study. The first limitation being the length of time of the study. Advancements in medicine have the ability to shift best practices and methods over time potentially affecting patient care and LOS. Another limitation was this was a single-center study. Additionally, the patients in this study were receiving care at a medical center in the Midwest, USA that mainly serves Medicare and Medicaid populations. The errors in the model may have introduced prediction error which could have under or over-predicted the treatment effect. The use of historical controls might have been a confounding factor. Finally, the physical movement of patients throughout the NICU hospitalization to different rooms or locations in the unit was not studied, only the team assignments, however nursing assignments were clustered based on acuity level, with the majority of low-acuity patients in physical proximity to one another.
Conclusions
This study demonstrated that the implementation of the acuity grouping model resulted in a significant reduction in NICU LOS. Clustering infants by acuity level might lead to infants with lower acuity status achieving more restful states to feed and grow, optimizing discharge readiness, when physically moved away from higher acuity areas. Additionally, having dedicated staffing assignments for lower acuity patients clustered together, similar to step-down rooms historically used in open bay NICUs, enhances discharge preparation as the staff has more time to focus on completion of discharge tasks and teaching for families. The time to complete discharge tasks and provide education and teaching for families would otherwise be limited if nursing assignments included mixed high and low acuity patients as more time is spent at the bedside of a high acuity infant. This was a single center study and further research is needed to evaluate multiple hospitals to limit the threat to validity. Further research is also needed to optimize NICU layouts. The accumulation of reduced days across hundreds of patients has the potential to reduce costs for both families and the hospital. The importance of such efforts has only been further emphasized by the recent coronavirus disease 2019 (COVID-19) pandemic, which has brought to light the need for resource optimization and efficient LOS.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jhmhp.amegroups.com/article/view/10.21037/jhmhp-23-71/rc
Data Sharing Statement: Available at https://jhmhp.amegroups.com/article/view/10.21037/jhmhp-23-71/dss
Peer Review File: Available at https://jhmhp.amegroups.com/article/view/10.21037/jhmhp-23-71/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jhmhp.amegroups.com/article/view/10.21037/jhmhp-23-71/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Rush University Institutional Review Board (ORA No. 20102202-IRB01) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Osterman MJK, Hamilton BE, Martin JA, et al. Births: Final Data for 2021. Natl Vital Stat Rep 2023;72:1-53. [PubMed]
- Phibbs CS, Schmitt SK, Cooper M, et al. Birth Hospitalization Costs and Days of Care for Mothers and Neonates in California, 2009-2011. J Pediatr 2019;204:118-125.e14. [Crossref] [PubMed]
- Waitzman NJ, Jalali A, Grosse SD. Preterm birth lifetime costs in the United States in 2016: An update. Semin Perinatol 2021;45:151390. [Crossref] [PubMed]
- White RD; Consensus Committee on Recommended Design Standards for Advanced Neonatal Care. Recommended standards for newborn ICU design, 9th edition. J Perinatol 2020;40:2-4.
- Noise: a hazard for the fetus and newborn. American Academy of Pediatrics. Committee on Environmental Health. Pediatrics 1997;100:724-7. [Crossref] [PubMed]
- Parra J, de Suremain A, Berne Audeoud F, et al. Sound levels in a neonatal intensive care unit significantly exceeded recommendations, especially inside incubators. Acta Paediatr 2017;106:1909-14. [Crossref] [PubMed]
- Arya R, Wei G, McCoy JV, et al. Decreasing length of stay in the emergency department with a split emergency severity index 3 patient flow model. Acad Emerg Med 2013;20:1171-9. [Crossref] [PubMed]
- Chou JH, Roumiantsev S, Singh R. PediTools Electronic Growth Chart Calculators: Applications in Clinical Care, Research, and Quality Improvement. J Med Internet Res 2020;22:e16204. [Crossref] [PubMed]
- Lakshmanan A, Song AY, Belfort MB, et al. The financial burden experienced by families of preterm infants after NICU discharge. J Perinatol 2022;42:223-30. [Crossref] [PubMed]
- Ionio C, Mascheroni E, Colombo C, et al. Stress and feelings in mothers and fathers in NICU: identifying risk factors for early interventions. Prim Health Care Res Dev 2019;20:e81. [Crossref] [PubMed]
- Montirosso R, Provenzi L, Calciolari G, et al. Measuring maternal stress and perceived support in 25 Italian NICUs. Acta Paediatr 2012;101:136-42. [Crossref] [PubMed]
- Santos J, Pearce SE, Stroustrup A. Impact of hospital-based environmental exposures on neurodevelopmental outcomes of preterm infants. Curr Opin Pediatr 2015;27:254-60. [Crossref] [PubMed]
Cite this article as: Seske LM, Sperry C, Selip DB, Carmignani KC, Cooper S, Johnson TJ. Aggregating patients for discharge readiness in the neonatal intensive care unit: impact on length of stay. J Hosp Manag Health Policy 2023;7:18.

