THRIVE intervention development: using participatory action research principles to guide a mHealth app-based intervention to improve oncology care
Introduction
Women with hormone receptor-positive (HR+) early-stage breast cancer (ESBC) are usually prescribed an adjuvant endocrine therapy (AET) medication after active treatment (e.g. , breast surgery, chemotherapy, radiation) to reduce the risk of cancer recurrence and mortality (1,2). Despite its efficacy, AET nonadherence is common due, in part, to adverse side effects (3-5). Women on AET frequently report negative symptoms, such as pain, fatigue, hot flashes, sexual dysfunction and mental health challenges, all of which can negatively impact quality of life during survivorship (6-8). Adverse side effects significantly contribute to AET nonadherence for Black women, which may account for mortality disparities (9).
Clear, patient-centered communication between a woman with ESBC and members of her oncology care team is essential for timely identification and management of AET-related side effects (10,11). Effective patient-provider communication can reduce medication nonadherence and poor patient health outcomes for breast cancer survivors (12,13). However, communication challenges in the clinical oncology setting persist (14,15), highlighting the need for novel methods. mHealth technologies (e.g. , web-enabled apps and devices) may offer innovative ways to improve healthcare service delivery and patient health outcomes.
mHealth apps have been employed to promote prevention, improve early detection, manage cancer care, and support cancer survivors (16,17). mHealth apps can also improve communication processes and information exchange between patients and providers (18). However, for mHealth interventions to have the potential to be effective at changing behavior and health outcomes, the app and its features must first engage users. Overall, a quarter of all apps downloaded to smart mobile devices are used only once (19). Engaging users/patients in the design process is critical for improving the likelihood of success of any mHealth intervention. To accomplish this, extensive formative work is recommended to test the interface and user experience among patients that would benefit from the intervention (19).
User-centered or participatory design, reflecting principles of participatory action research (PAR), is characterized by involving end users as co-developers in design phases and is recognized as an essential aspect of good mHealth design practice (19,20). mHealth interventions developed in accordance with PAR principles (e.g. , patient empowerment, equitable, sustained participation by community stakeholders) increase relevance, appropriateness, rigor, validity and sustainability (21). Still, few studies have examined patient user preferences or solicited feedback from the healthcare team tasked with implementing the intervention. To avoid potential pitfalls from lack of stakeholder engagement in mHealth intervention development, we applied PAR principles in conducting focus groups and interviews to refine and enhance a web-enabled app-based intervention to improve symptom management and medication adherence for women with ESBC on AET. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/jhmhp-20-103).
Description of the THRIVE intervention
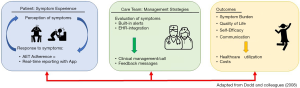
The THRIVE intervention aims to facilitate timely and responsive symptom management and improve medication adherence by collecting and transmitting patient-reported symptoms and other potential adherence barriers to medical oncology care teams. The intervention is guided by the symptom management model (Figure 1), which describes the interrelatedness of three symptom management dimensions: symptom experience, management strategies, and health outcomes (23). The model is based on the assumptions that the patient’s perception of symptoms is the gold-standard of measurement; troublesome symptoms must be monitored and managed in a timely manner, and symptom management is dynamic (23).
In our pilot trial, we tested the feasibility of THRIVE among 44 women with HR+ ESBC and a new prescription for an AET medication. THRIVE uses a web-enabled app designed to improve patient-provider communication about AET adherence and related adverse symptoms outside of clinic visits. Intervention group participants received weekly reminders to report adherence in the last seven days and any new or changing adverse symptoms for eight weeks. Built-in, real-time alerts and electronic health record-integration allows any patient’s report of AET nonadherence or adverse symptoms to be continually evaluated by the patient’s oncology team and promptly address symptoms and other patient concerns, resulting in improved patient symptom experience and AET adherence. Participants randomized to receive weekly reminders to use the study app self-reported significantly higher AET adherence at eight weeks compared with controls (91% vs. 68%, P=0.02) (24). We are in the process of testing an updated version of the THRIVE intervention that builds on the success of the pilot study by: (I) engaging patients in the design and content of the app, text messages, and recruitment protocols-the focus of this paper; (II) including a larger sample, stratified by race, powered to test the intervention with and without tailed feedback messages; (III) expanding the intervention period from two to six months in order to capture later-onset adverse symptoms that might be slower to develop; and (IV) following participants for one to three years to test longer-term effects of the intervention on medication adherence and other outcomes. We are currently randomizing 300 patients initiating AET to one of three arms: (I) an “App” group (N=100) that receives weekly reminders to use the THRIVE study app; (II) an “App+Feedback” group (N=100) that receives weekly reminders and tailored feedback based on their use of the app; or (III) a “Usual Care” group (N=100) that receives usual care only (22). The primary outcome is medication adherence at 12 months, which is captured using an electronic monitoring pillbox.
Methods
The West Cancer Center and Research Institute (WCCRI), our partner for this study, provides a network of fully integrated cancer care at 9 clinic locations in Tennessee, Arkansas, and Mississippi. The WCCRI treats more than 1,200 patients with a new breast cancer diagnosis annually.
Patient focus groups
To update and refine the THRIVE intervention, including the web-enabled app, and ensure its relevance to the target audience, we conducted four focus groups involving women with ESBC on AET, stratified by race (Black and White) and length of time on AET (<6 and >6 months). Adult women (ages 18 years and older) with a diagnosis of HR+ ESBC and a prescription for an AET medication, including tamoxifen or aromatase inhibitor, were eligible. To recruit focus group participants, WCCRI physicians (LS and GV) identified potential participants from their patient rosters, and the study nurse (TJ) reviewed electronic health records. TJ contacted women by telephone, reviewed the study procedures, answered questions, and confirmed their eligibility.
Those who were interested in study participation were scheduled for a one-time focus group at the WCCRI campus. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the University of Tennessee Health Science Center Institutional Review Board (17-05479-XP IAA) and informed consent was provided by all of the study participants. Demographic information (e.g. , race, age, income, educational level) and cancer-related personal characteristics (e.g. , staging at initial breast cancer diagnosis, family history, AET prescription) were collected from focus group participants via a brief questionnaire.
Focus groups were moderated by race-concordant moderators (JA and RK). Single-race focus groups were used to create opportunities for breast cancer survivors to engage in open dialogue with their peers in safe, nonjudgmental environments. Focus group participants were prompted to share their insights about patient-provider communication and technology in healthcare (14) and essential components and functional requirements of the THRIVE app, as well as to assess acceptability of app message content and frequency of reminder messages. Moderator guide used for these four focus groups included in Appendix 1.
A fifth mixed-race focus group was convened (N=6), comprised of participants from previous focus groups, to provide feedback on THRIVE app content using high-fidelity mock-ups and changes that would make the app more helpful and engaging. Participants were shown tailored feedback messages that focused on patient-reported AET adherence and symptoms, positive reinforcement, and motivational messages, and they were asked to provide feedback on messages that would lead to optimal adherence. The Moderator Guide used in this focus group is included in Appendix 2. Using a scale of 1–5, they evaluated each message for attention, motivation, personal relevance, appropriateness, and acceptability and decided which affirmations would be included in the intervention (Table 1). Lastly, participants were asked to select the electronic pill monitor to be used in the study and identify incentives that would facilitate participant retention. All focus group participants were compensated with a $40 gift card for each focus group in which they participated.

Full table
Nurse interviews
Semi-structured, in-person interviews were conducted with oncology nurses (N=5) charged with implementing the THRIVE intervention in clinical practice to qualitatively assess the impact of the THRIVE app on patient-provider communication, provider responsiveness to patients’ adverse symptoms, clinical workflow, and initial challenges to app implementation. TJ recruited eligible nurses, and JA interviewed each nurse on the WCCRI campus. Interviews lasted approximately 20 minutes, and nurse participants were compensated with a $25 gift card. All nurse participants self-identified as female; three nurse participants were White and two were Black.
Data analysis
Focus group sessions were audio recorded and professionally transcribed verbatim. Transcripts were imported into NVivo 11Plus (QSR International Pty Ltd. ). Researchers (JA and CG) read each transcript and conducted line-by-line coding independently. They engaged in an iterative process of developing codes for each focus group, discussing differences to clarify codes names and descriptions, and referencing transcript texts and audio recordings to resolve differences. Final analysis yielded themes as well as categories and sub-categories for themes. RK assessed internal validity and reliability by independently examining thematic analyses for clarity, consistency, credibility, and meaning (25,26).
Results
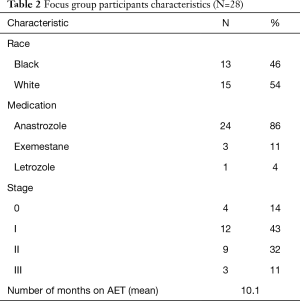
Twenty-eight women (average age: 64 years) took part in the focus groups. Most had stage I or stage II breast cancer at diagnosis (43% and 32%, respectively). Early AET users averaged 2.63 months on their prescribed medication; late AET users averaged 16.55 months (Table 2). Participants in the four initial focus groups expressed their preferences for app features that would aid them in identifying and self-monitoring AET-related symptoms (i.e. , body map, free-text option, patient dashboard, social support resources); content for tailored feedback messages and positive affirmations; and desired frequency of reminder messages to use the THRIVE app. Participants in the 5th focus group indicated their preference for an electronic pillbox and a loss-framed incentive plan to be used in the THRIVE intervention. Participants’ responses about preferences for app content and features were compared to see if any race-based differences emerged; no substantive differences were found.
Full table
Nurses expressed favorable opinions of the THRIVE app, stating it improved patient-provider communication between patients and members of their oncology team. The app also improved providers’ abilities to quickly address a patient’s concerns about adverse symptoms and AET adherence challenges. Nurses reported the THRIVE app and alerts did not interrupt clinical workflow or increase work burden.
THRIVE app features
Body map
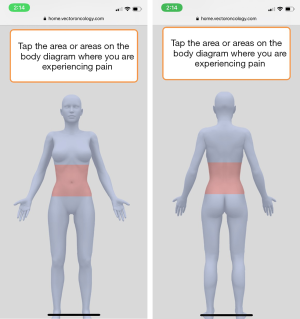
Most participants in the initial focus groups described experiencing pain; however, some shared that they often have difficulty describing multiple pain locations with varying severity levels. Participants reported wanting a way to delineate their specific pains beyond a numeric rating scale: “Will we have something to list our type of pain we are having? Because this is just general, saying that I have pain, but you won’t know what kind of pain I am having. ” To address participants’ concerns about potential miscommunication with providers, a body map was added to the THRIVE app. The body map features anterior and posterior views of a female figure, and users can select any part of the body to indicate a pain site (Figure 2). Users are also able to rate the pain severity on a scale of 1–10 (10 being most severe). Participants in the 5th focus group reviewed the mock-up of the body map for the THRIVE app and gave it unanimously positive feedback. One participant said, “It’s awesome,” and another woman said, “Yes! Because it is hard to tell someone exactly where you are talking about it hurts. ”
Free-text option
In three focus groups, participants discussed how AET medications can affect every woman differently, which prompted several women to seek clarification about the “other” response option. A few women explicitly asked if an open-response box would be included in the THRIVE app. One participant said, “Is there something for you to fill out for yourself that is not on the list? Like if I have a side effect that is not on this list that would be ‘other’?” Another participant queried: “If you hit ‘other,’ does it go to give you other reasons or give you a fill in box?” Participants insisted it would be critical to their ability to describe symptoms and communicate with their provider accurately. Based on this feedback, the study team engaged our technology partner to ensure a free-text option would meet patient needs without creating unnecessary provider burden from uncategorizable—and potentially unactionable—patient-reported information. The free-text option was then placed at the end of the decision tree to ensure that information recorded in the free-text section included additional, unique information not recorded in previous steps (Figure 3). All participants in the fifth focus group positively rated the free-text option.
Patient dashboard
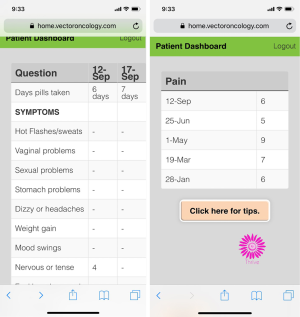
Several breast cancer survivors in the initial four focus groups shared that they use the electronic patient portal to track their lab results and monitor their treatment progress. One participant said, “To always have those at your fingertips if I want to go back and see what my lab was on that day and how this was or that was, that is good to have that. ” Participants indicated that it would be helpful to track AET-related symptoms on the app in a similar way: “I think that it would also be helpful, at least for me, to know what days I have certain symptoms because maybe I am doing something on those days to make my symptoms worse. Give me an idea that maybe what I am eating or doing is aggravating this. ” To address this point, a dashboard feature displays dates when symptoms were reported, so patients can track their symptoms in real-time, see how symptoms change with time, and report trends to their oncology provider (Figure 4).
Social support resources
In each of the initial four focus groups, participants expressed the importance of providing within-app social support resources. Several participants acknowledged that patients receive written materials when they are first diagnosed with cancer, but if those resources are not readily accessible, then patients’ social support needs may remain unmet. To address participants’ suggestions, the THRIVE app features a hyperlink to the WCCRI website that contains a list of local and national support organizations.
Patient-friendly language
Focus group participants reported their desires for message wording to be both personal and professional. Participants expressed an understanding that the app would be used for medical purposes in a clinic setting; however, they reported wanting language that was “a little bit more emotional, less like something that’s just coming off of a computer. ” Additionally, participants wanted “cancer” removed from app messages. In one focus group, several participants brainstormed appropriate alternatives for “the C-word”:
Participant A: I don’t like having anti-hormonal cancer medicine said over again. I know that’s what it is and I know that that’s what the survey is, but couldn’t we call it something else, something nice?
Participant B: Maybe like cancer medication?
Participant C: Hormone blocker? That’s what I always call it just a hormone blocker.
Moderator: So that would be more positive language?
Participant C: Instead of throwing “cancer” in there every time.
Participant A: Yes, I know it’s there.
Participant C: Yeah, we know it. We have all went through it. Yeah, we know what it is.
This dialogue between focus group participants illustrates how breast cancer survivors find alternative medical words or phrases (e.g. , medication, hormone blocker) for the word “cancer” to avoid frequent reminders about their diagnosis.
App aesthetics
A few participants openly shared their dislike of the THRIVE app’s initial muted green color palette. One participant said, “For me I like colorful things…colorful things kinda bring it out. ” Another participant asked, “Why don’t we have pink?” and indicated a preference for signature breast cancer pink to which other participants agreed. Based on this feedback, the THRIVE logo and icon app color scheme was changed to bold pink (Figure 5).

THRIVE intervention characteristics
Self-affirmation messages
Study team members generated a list of self-affirmations that patients in the “App” intervention group would receive weekly. Each self-affirmation message includes a link to the THRIVE app. Breast cancer survivors in the initial four focus groups reported positive perceptions of the statements: “I get so tickled because I don’t like apps, but I had to give all five stars because they are good. I think they was encouraging because some days I need it. ”
Tailored feedback messages
Overall, focus group participants expressed positive views of the THRIVE app and its features, particularly the app’s potential to improve patient-provider communication and AET adherence. One participant said, “I like the idea that you can get messages back based on what you put into the survey, instead of feeling like sending information off into the air. ” Another said, “The personalized feedback gives you a feeling that you are connected instead of just ‘here you are one month and then again six months later’, so more interaction with your healthcare team. ” Black and White women did not differ in their expressed desires for THRIVE app content and features; however, Black women in both single-race focus groups reported wanting an option for daily reminders to take their AET medication, especially for those with new prescriptions. One participant said, “If every time I clicked on my phone and it say, ‘Did you take your med?’ then I say, ‘Okay. Let me go take my medication. ’ I’m gonna stop and do that. ” Black women also reported wanting timed medication messages (e.g. , morning, afternoon, or evening) with graphics as personalized, visual reminders to adhere to their AET regimens. For instance, one participant said, “Don’t send no words just send a little pill bottle in that app, and then we will say, ‘let’s go twist that bottle. ’”
A pool of 126 tailored messages for study participants in the “App+Feedback” group were developed based on patient characteristics, including self-reported AET-related symptoms, lifestyle and preferred activities or hobbies, and religion/spirituality. Messages were also tailored to reflect the name of a patient’s AET medication and provider. Several participants reported liking the potential just-in-time nature of tailored feedback messages, especially if they experience mental health challenges because of their cancer diagnosis or as a side effect of the AET medication. One participant said, “You never know when you might just get that just at the right moment. You know, like, ‘Wow! That was a God thing. The Lord took care of that. ’” Additionally, focus group participants identified additional AET-related symptoms, specifically sleep disturbances/insomnia and dental health challenges (e.g. , soft tooth enamel, tooth cracking, and tooth loss), which were subsequently added.
Participants in the fifth focus group worked in pairs to develop original feedback messages for the THRIVE intervention that highlighted the importance of adhering to one’s AET regimen, engaging in open communication with healthcare providers, and maintaining an active, healthy lifestyle for one’s self and others. At the end of the workshop session, each pair of survivors shared their feedback messages with the larger group. Participants engaged in back-and-forth dialogue to ensure the content of each new message was relevant to the objectives of the THRIVE intervention and spoke to the experiences of breast cancer survivors taking AET. Examples of survivor-generated messages that were included in the THRIVE intervention are: “AET doesn’t work if you don’t take it. Make it your goal this week to take your meds exactly how the doctor prescribed” and “Don’t hesitate to report uncomfortable side effects. Your physician is not bothered by your calls. ” Table 3 provides examples of tailored feedback messages. Focus group participants also reported liking messages with appropriate, related images; thus, tailored feedback messages with race-concordant graphics were added (Figure 6).

Full table

Reminder frequency
Focus group participants expressed belief that the THRIVE app and reminder messages would aid them in mindfulness about their cancer diagnosis and the importance of AET medication adherence. For instance, one participant said the THRIVE intervention would help women with breast cancer to “remember to not just put your head in the sand and say, ‘I don’t want to do this anymore. ’” Another participant said, “I think that it helps you become more accountable and to do your part. If you are doing well, you don’t just stuff it (cancer diagnosis) back. You’ll remember that this is the reality of it. You are reminded to remember that this could re-occur so continue to do what you are doing even if it was several years back. ”
In each of the first four focus groups, participants reported their preferences for reminder messages to use the THRIVE app. Participants expressed unanimous displeasure for daily reminder messages to use the app. Some indicated that, receiving reminder messages too frequently could cause “irritation” and prompt them to turn off app notifications. The majority of participants expressed a preference for weekly reminder messages. One participant said, “I would say once a week. Don’t bounce me every day. ” Another participant said, “So I won’t remember if I’m supposed to do it every week and I only get a message once a month. ” Focus group participants suggested that reminder message frequency should be adjusted to a patient’s length of time on AET, such that patients with new prescriptions would receive reminder messages with greater frequency than those who had been on AET for longer periods. For instance, one participant said, “I am thinking to begin with that weekly may be good but then once you have been on the medication for a while then weekly is going to seem like ‘Oh my gosh. I just did this, and do I have to do it again!’ So maybe monthly after that, a progressive schedule. ” Unfortunately, researchers were unable to address this suggestion given limitations of the existing technology.
Electronic pill monitoring
A key component of the THRIVE intervention is using an electronic device to objectively evaluate the effect of the THRIVE app and messaging on breast cancer survivors’ medication adherence. Participants in our fifth focus group were asked to identify their preference for an electronic pill monitor from two options. The first option was a pill bottle with an electronic cap that recorded each time the bottle was opened. This option required patients to own a smart phone and download an app (in addition to the THRIVE app) that captured and stored data from the electronic cap. The second option was a device (model RT2000) that resembled a pillbox developed by Wisepill. Data was captured each time the device was opened without patients having to download an additional app. Prior to selecting a preferred option, focus group participants asked about any negative effects of the electronic devices on the integrity of their AET medication, “Do pills lose their strength in here?” and debated the pros and cons of each option. For instance, one participant said, “The only thing I like about the bottle (option 1) is that you can put entire prescription in there and that you don’t have to worry about refill your pill boxes because you have to remember to refill the pill boxes. ” One participant said she preferred the pillbox “because that is what I do now. ” There was not consensus among participants, yet the majority (4 out of 6) indicated a preference for the pillbox (i.e. , second option) (Figure 7).
Incentive schedule for research participants
Participants in the fifth focus group were also asked to provide feedback on potential incentive plans for THRIVE intervention participants. Women in this focus group were presented with three options: two gain-framed plans and one loss-framed plan. The first option incentivized THRIVE intervention participants with a $5 monthly credit for study compliance (e.g. , storing AET medication in the electronic pill monitor, keeping device charged); in the second option, study participants were given the same $5 monthly credit for study compliance plus one entry into a monthly lottery for a chance to earn an additional $20 or $300. The third option, the loss-framed plan, incentivized intervention participants with an initial $60 credit from which $5 monthly deductions would be taken for study noncompliance. One participant explained why she thought the gain-framed lottery option was unfair: “That lottery thing, no. Because what if I am really good about doing mine and someone else is halfway doing theirs? You know, they might do it, but I’m just better and I take more time and I care more, but they’re still up for the same incentive. ” Focus group participants unanimously selected the loss-framed incentive plan, as one participant said, “Because you know it’s there, and you gotta keep it. ”
Implementation feedback from WCCRI oncology nurses
Nurses reported favorable perceptions about the role of technology in healthcare service delivery. Several nurses said that technologies like web-enabled apps and patient portals give their patients more opportunities to share valuable information with healthcare providers who, in turn, are able to provide tailored, responsive feedback and better care. Yet, one nurse participant did express some reservations about technology, saying “technology can be beneficial” but may create additional barriers for older or low-income patients.
Every nurse participant reported initial reservations about the impact of the THRIVE app on clinical workflow and work burden. One nurse said, “I have to say, initially I thought it was going to be a big burden. ‘Oh my gosh! I don’t want to get all this extra communication. ’” However, all but one nurse reported THRIVE alerts did not increase work burden, and each nurse participant reported the app increased the potential for patient-provider communication. Specifically, nurses said the new free-text feature would be “helpful” because it increased the amount of information nurses had to address treatment issues. Nurses also reported that the THRIVE app gives patients more direct access to their oncologists and his/her care team, especially for patients who do not have direct contact with their nurse (e.g. , personal email or cell phone). Additionally, nurses reported that patients often feel like they are bothering their providers if they contact them to report negative symptoms or challenges with medication adherence. They believe that since patients who use the THRIVE app are able to report their symptoms in real-time, nurses are able to intervene quickly when a patient is thinking about stopping AET medication because of adverse side effects. Nurse participants said THRIVE alerts can increase communication between the first AET prescription and 8-week follow-up visit, so patients’ symptoms can be addressed quickly and “patients don’t have to suffer. ” Nurses in this study reported no negative feedback or usability concerns with the THRIVE app. All nurses said they were comfortable with the intervention protocol and would recommend app-based interventions like THRIVE to other oncology nurses.
Discussion
Most women with ESBC on AET experience multiple adverse effects that can contribute to AET nonadherence, and they desire timely, patient-centered communication with oncology team members to manage AET-related symptoms and maintain their quality of life. This study describes development of the THRIVE intervention to demonstrate how an iterative, user-centered process, guided by PAR principles (21), can be employed to design a culturally and contextually relevant mHealth intervention that improves healthcare provision. Findings from this qualitative inquiry engaged patients and nurses to provide input on all key elements of the THRIVE intervention. We revised the THRIVE app content by incorporating patient-requested app features, app aesthetics, and message content. The app has the potential to improve AET adherence and quality of life among breast cancer survivors and reduce disparities in mortality rates for Black women by facilitating bidirectional communication with healthcare providers (27).
Black women face unique communication challenges related to side effects that contribute to AET nonadherence (14,28,29), which may account for mortality disparities. Thus, it was essential that we engaged in a culturally tailored approach to develop a mHelath intervention. We engaged Black breast cancer survivors in single- and mixed-race focus groups to better understand their unique needs and preferences for technology-facilitated patient-provider communication and healthcare provision. THRIVE app features (e.g. , tailored feedback messages with race-concordant graphics and culturally appropriate language) are responsive to suggestions from our patient experts. Little is known about unique preferences among Black women with ESBC for technology “touches” (e.g. , medication reminder messages) in app-based interventions, highlighting an additional area of mHealth research. Our participatory approach allowed us to go beyond a surface-level view of the scope and nature of Black breast cancer survivors’ challenges and barriers to develop an mHealth solution that can circumvent issues that exacerbate health inequities (30).
Additionally, initial focus groups were stratified by duration of AET use to generate participant feedback that would better reflect the changing needs and experiences of women on AET. Recommendations that emerged from these discussions, such as providing links to WCCRI resources and support groups, may also help fill in support gaps experienced among many patients, following a period of decreased social support from patient support networks when compared to the primary phase of treatment (31,32). Given the trend of decreasing patient-provider interactions during the adjuvant treatment phase, enabling patients to utilize technology to report and seek recommendations for potentially severe AET-related side effects outside of the clinic visit may prevent early AET discontinuation.
In a recent study, healthcare providers reported favorable perceptions of telemedicine, particularly mobile apps, in oncology care for their abilities to improve patient-provider communication and facilitate patient reminders for medication intake (33). Yet, most breast cancer-focused mHealth apps lack healthcare professional involvement in development (34). The THRIVE intervention was developed in collaboration with WCCRI oncologists and nurses to ensure the app contains functionalities that would improve providers’ abilities to address a patient’s concern in a timely manner without disrupting clinical workflow or adding to their work obligations. WCCRI nurses in our study reported one of the hallmarks of the THRIVE app is the ability for nursing staff to quickly respond to symptom alerts, thereby reducing the likelihood of patient AET nonadherence.
Despite the strengths of this study, there are some limitations worth mentioning. As with most qualitative methods, generalizability of this study’s findings to other populations is limited by several factors. Given that our patient population is women with ESBC, these patient preferences might not be the same for other disease profiles of differing severity. Women with breast cancer metastases are likely to have different concerns and express different preferences for an app. More research is needed to understand how patients with later stages or greater disease burden perceive usability and benefit of the intervention. Furthermore, our sample was relatively small and also drawn from a single healthcare facility in the United States Midsouth; thus, certain factors (e.g. , nurses’ perceptions of work burden from app alerts) might vary as a function of existing staff workload and institutional infrastructure. Though our study captures the views of a diverse set of Black and White women with ESBC, preferences about app features and other intervention-related decisions (e.g. , incentive scheme, pillbox preferences) might differ across more robust sociocultural, generational, geographic, and racial/ethnic backgrounds.
We are still in the process of testing the effectiveness of the THRIVE intervention on medication adherence and other key outcomes (22). This research adds to our understanding of how patients perceive and use a web-enabled app to further engage with their care team outside of clinic visits. Considering how ubiquitous web-enabled mobile devices have become, a successful intervention could be disseminated across health systems, conditions, and populations. Our results describe the process of applying PAR principles to tailor an interactive mHealth interventions. This process can be applied to other mHealth interventions to improve their effectiveness in delivering a low-cost and scalable intervention to improve outcomes across diverse patient populations and settings.
Acknowledgments
Funding: A grant from the National Cancer Institute (R01CA218155) provided support for this research study.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Naleef Fareed, Ann Scheck McAlearney, and Susan D Moffatt-Bruce) for the series “Innovations and Practices that Influence Patient-Centered Health Care Delivery” published in Journal of Hospital Management and Health Policy. The article has undergone external peer review.
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/jhmhp-20-103
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jhmhp-20-103
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jhmhp-20-103). The series “Innovations and Practices that Influence Patient-Centered Health Care Delivery” was commissioned by the editorial office without any funding or sponsorship. Dr. GAV reports receiving personal fees from Roche/Genetech, Novartis, Eli Lilly, Immunomedics, Puma, Pfizer, AstraZeneca, Biotheranautics, Daiichi Sankyo, Vector Oncology and research funding from Roche/Genetech, Puma, Celcuity, Merck, BMS, Eli Lilly, GTx inc, Astrazeneca, Pfizer, Immunomedics, Tesaro, Halozyme, and ownership of Oncodisc, outside the submitted work. Dr. LS reports receiving personal fees from Amgen, Pfizer, Helsinn, Genentech, Genomic Health, BMS, Myriad, AstraZeneca, Bayer, Spectrum, Napo and research support from Amgen, Pfizer, outside the submitted work. Dr. IG reports receiving research support from Pfizer, outside the submitted work. Dr. RAK reports a speaking fee from General Mills, Inc, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the University of Tennessee Health Science Center Institutional Review Board (17-05479-XP IAA) and informed consent was taken from all the study participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. J Clin Oncol 2016;34:1689-701. [Crossref] [PubMed]
- Makubate B, Donnan PT, Dewar JA, et al. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer 2013;108:1515-24. [Crossref] [PubMed]
- Lambert LK, Balneaves LG, Howard AF, et al. Patient-reported factors associated with adherence to adjuvant endocrine therapy after breast cancer: an integrative review. Breast Cancer Res Treat 2018;167:615-33. [Crossref] [PubMed]
- Murphy CC, Bartholomew LK, Carpentier MY, et al. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat 2012;134:459-78. [Crossref] [PubMed]
- Cahir C, Guinan E, Dombrowski SU, et al. Identifying the determinants of adjuvant hormonal therapy medication taking behaviour in women with stages I-III breast cancer: A systematic review and meta-analysis. Patient Educ Couns 2015;S0738-3991(15)00234-7.
- Ganz PA, Petersen L, Bower JE, et al. Impact of Adjuvant Endocrine Therapy on Quality of Life and Symptoms: Observational Data Over 12 Months From the Mind-Body Study. J Clin Oncol 2016;34:816-24. [Crossref] [PubMed]
- Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat 2008;107:167-80. [Crossref] [PubMed]
- Mouridsen HT. Incidence and management of side effects associated with aromatase inhibitors in the adjuvant treatment of breast cancer in postmenopausal women. Curr Med Res Opin 2006;22:1609-21. [Crossref] [PubMed]
- Wheeler SB, Spencer J, Pinheiro LC, et al. Endocrine Therapy Nonadherence and Discontinuation in Black and White Women. J Natl Cancer Inst 2019;111:498-508. [Crossref] [PubMed]
- Turner K, Samuel CA, Donovan HA, et al. Provider perspectives on patient-provider communication for adjuvant endocrine therapy symptom management. Support Care Cancer 2017;25:1055-61. [Crossref] [PubMed]
- Van Liew JR, Christensen AJ, de Moor JS. Psychosocial factors in adjuvant hormone therapy for breast cancer: an emerging context for adherence research. J Cancer Surviv 2014;8:521-31. [Crossref] [PubMed]
- Liu Y, Malin JL, Diamant AL, et al. Adherence to adjuvant hormone therapy in low-income women with breast cancer: the role of provider-patient communication. Breast Cancer Res Treat 2013;137:829-36. [Crossref] [PubMed]
- Sheppard VB, Adams IF, Lamdan R, et al. The role of patient-provider communication for black women making decisions about breast cancer treatment. Psychooncology 2011;20:1309-16. [Crossref] [PubMed]
- Anderson JN, Graff JC, Krukowski RA, et al. "Nobody Will Tell You. You’ve Got to Ask!": An Examination of Patient-provider Communication Needs and Preferences among Black and White Women with Early-stage Breast Cancer. Health Commun 2020.1-12. [Crossref] [PubMed]
- Li CC, Matthews AK, Dossaji M, et al. The Relationship of Patient-Provider Communication on Quality of Life among African-American and White Cancer Survivors. J Health Commun 2017;22:584-92. [Crossref] [PubMed]
- Bender JL, Yue RY, To MJ, et al. A lot of action, but not in the right direction: systematic review and content analysis of smartphone applications for the prevention, detection, and management of cancer. J Med Internet Res 2013;15:e287. [Crossref] [PubMed]
- Mobasheri MH, Johnston M, King D, et al. Smartphone breast applications - what’s the evidence? Breast 2014;23:683-9. [Crossref] [PubMed]
- Jongerius C, Russo S, Mazzocco K, et al. Research-Tested Mobile Apps for Breast Cancer Care: Systematic Review. JMIR Mhealth Uhealth 2019;7:e10930. [Crossref] [PubMed]
- McCurdie T, Taneva S, Casselman M, et al. mHealth consumer apps: the case for user-centered design. Biomed Instrum Technol 2012.Suppl:49-56. [Crossref] [PubMed]
- Kushniruk A, Nohr C. Participatory Design, User Involvement and Health IT Evaluation. Stud Health Technol Inform 2016;222:139-51. [PubMed]
- Gerhardt U, Breitschwerdt R, Thomas O. Engineering sustainable mHealth: the role of Action Research. AI & SOCIETY 2017;32:339-57. [Crossref]
- Paladino AJ, Anderson JN, Krukowski RA, et al. THRIVE study protocol: a randomized controlled trial evaluating a web-based app and tailored messages to improve adherence to adjuvant endocrine therapy among women with breast cancer. BMC Health Serv Res 2019;19:977. [Crossref] [PubMed]
- Dodd M, Janson S, Facione N, et al. Advancing the science of symptom management. J Adv Nurs 2001;33:668-76. [Crossref] [PubMed]
- Graetz I, McKillop CN, Stepanski E, et al. Use of a web-based app to improve breast cancer symptom management and adherence for aromatase inhibitors: a randomized controlled feasibility trial. J Cancer Surviv 2018;12:431-40. [Crossref] [PubMed]
- Leavy P. The Oxford handbook of qualitative research. USA: Oxford University Press, 2014.
- Lincoln YS, Guba EG. Naturalistic inquiry (vol. 75). CA: Sage Thousand Oaks, 1985.
- Finitsis DJ, Vose BA, Mahalak JG, et al. Interventions to promote adherence to endocrine therapy among breast cancer survivors: A meta-analysis. Psycho-oncology 2019;28:255-63. [Crossref] [PubMed]
- White-Means SI, Osmani AR. Racial and Ethnic Disparities in Patient-Provider Communication With Breast Cancer Patients: Evidence From 2011 MEPS and Experiences With Cancer Supplement. Inquiry 2017;54:46958017727104. [Crossref] [PubMed]
- Roberts MC, Wheeler SB, Reeder-Hayes K. Racial/Ethnic and socioeconomic disparities in endocrine therapy adherence in breast cancer: a systematic review. Am J Public Health 2015;105 Suppl 3:e4-15. [Crossref] [PubMed]
- Stowell E, Lyson MC, Saksono H, et al. , editors. Designing and evaluating mHealth interventions for vulnerable populations: A systematic review. Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems; 2018.
- Paladino AJ, Anderson JN, Graff JC, et al. A qualitative exploration of race-based differences in social support needs of diverse women with breast cancer on adjuvant therapy. Psychooncology 2019;28:570-6. [Crossref] [PubMed]
- Thompson T, Rodebaugh TL, Perez M, et al. Perceived social support change in patients with early stage breast cancer and controls. Health Psychol 2013;32:886-95. [Crossref] [PubMed]
- Kessel KA, Vogel MME, Schmidt-Graf F, et al. Mobile Apps in Oncology: A Survey on Health Care Professionals’ Attitude Toward Telemedicine, mHealth, and Oncological Apps. J Med Internet Res 2016;18:e312. [Crossref] [PubMed]
- Giunti G, Giunta DH, Guisado-Fernandez E, et al. A biopsy of Breast Cancer mobile applications: state of the practice review. Int J Med Inform 2018;110:1-9. [Crossref] [PubMed]
Cite this article as: Anderson JN, Krukowski RA, Paladino AJ, Graff JC, Schwartzberg L, Curry AN, Vidal GA, Jones TN, Waters TM, Graetz I. THRIVE intervention development: using participatory action research principles to guide a mHealth app-based intervention to improve oncology care. J Hosp Manag Health Policy 2021;5:5.