Factors in sustained compliance to a symptom-reporting mobile application: implications for clinical implementation
Introduction
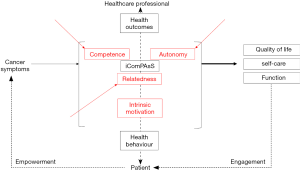
The Internet-based Computerized Patient Assessment System (iComPAsS) is a telemedicine application that allows patients to report their pain and symptoms levels regularly so that the healthcare professional can manage them more efficiently, leading to better quality of life and functional outcomes. This shifts the paradigm of symptom management back to the cancer patient, fostering empowerment over his symptoms through the mobile application. Situated within the therapeutic relationship between the patient and the healthcare professional, the iComPAsS aims to capitalize on the intrinsic motivation of the patient by addressing the three elements defined in the self-determination theory (1) (Figure 1).
The self-determination theory, commonly used in intervention programs and in the development of technological advancements outside (2) and within the healthcare system (such as medication adherence, smoking cessation, weight management, and lifestyle change programs) (3-8), targets optimal motivation that is characterized by three important elements—autonomy, or self-regulation of behavior according to interest, values or satisfaction with the behavior itself, rather than external reinforcement; competence, or the sense of capability to achieve one’s goals or desired outcomes in optimally challenging situations; and relatedness, or the degree to which one feels connected to and understood by others. Integration of these elements in a program or tool results in volitional or patient-initiated direction, which results in long-term and sustainable effects, compared to controlled-type motivation that is merely a result of pressure or demands from the healthcare professionals (8,9).
The iComPAsS was compared to usual pain care in a randomized clinical trial to evaluate its impact on pain control. The primary objective of this analysis was to examine the factors influencing patient compliance to a remote symptom-reporting mobile application use in order to gauge the sustainability of its effects and to help direct further development of the application and its eventual implementation as part of usual care.
Methods
Development of the mobile application
The iComPAsS, a tool for patient self-reporting of pain and symptoms, allows patients to report the severity of their pain and symptoms through the Internet using mobile phone, and physicians to view patient entries and send instructions back (e.g., to pick up a refill prescription or a revised prescription for purposes of dose titration, to follow-up in the clinic) and to generate graphical or statistical summaries (e.g., trend of pain over time from patient responses). A later design incorporated notifications that signaled patients to report at a frequency set by the physician based on the severity of pain, for purposes of dose titration.
Features of the mobile application
The Patient Interface is accessible from a mobile application, and is available in Android and iOS versions. The interface allows the patient to login, modify his username and password, access a menu, and log-out. The menu consists of the following options: Profile, Prescriptions, MyTools, List of Doctors, Messages, Change Password and Help. Profile allows the patient to view his demographic data and call the Pain Unit Hotline if data needs updating or correction; Prescriptions, to view physician instructions or prescriptions; MyTools, to access the Edmonton Symptom Assessment Scale (ESAS); List of Doctors to view a list of all his attending physicians, their specialties, schedules and office telephone numbers; Messages, to view instructions or messages from physicians and administrators; Change Password to change current password; and Help to access textual and a video tutorial on use, navigation and troubleshooting.
The Physician Interface allows the physician to login, modify his username and password, and access a menu, and log-out. The menu consists of the following options: Profile, List of Patients, Messages, Change Password, and Help. Profile allows the physician to view his profile (schedule, contact numbers, specialization) and to contact the administrator for necessary updates or corrections; List of Patients, to view a list of his patients, access their individual records (such as demographic data, clinical data, other attending physicians, and responses to ESAS or other tools); Messages, to view messages that he has sent to patients or coming from administrators, and to send messages to patients, such as reminders, instructions, prescriptions, and appointment schedules; Change Password to change current password; and Help to access textual and a video tutorial on use, navigation and troubleshooting of the application.
Trial design
The iComPAsS was compared to standard care in a parallel, randomized controlled trial. From April 2016 to May 2017, patients fulfilling the following criteria were recruited and included in the study: at least 18 years old, cancer diagnosis, pain score ≥4 on the Visual Analogue Scale, access to an Android or IOS phone, willing to learn and use a telemedicine application for symptom reporting, and no evidence of cognitive impairment or psychopathology. Recruitment of at least 33 patients on each arm was targeted. A written informed consent form was obtained.
Participants were randomized to either the standard care arm or the iComPAsS in a 1:1 ratio, thus allowing detection of statistical significance with the smallest number of participants. Cluster randomization at the physician level was done to reduce the risk of experimental contamination and, to achieve a better group balance, minimization, a form of adaptive randomization, was employed, using the following factors: sex, stage of disease, and payer status (charity case versus self-pay).
At our institute, standard pain management, as defined in the USTH BCI Policy Manual and the USTH Pain Management and Palliative Care Unit Procedural Manual, the registered pain nurse initially assesses the patient, using tools such as the ESAS along with other relevant health outcome or quality-of-life questionnaires. The findings are discussed with a pain management specialist, who then takes a medical and psychosocial history and conducts a directed physical examination. Patients presenting with complex problems are seen in a multidisciplinary meeting with other health care professionals (such as medical oncologist, radiation oncologist, surgical oncologist, orthopedic surgeon, palliative care specialist, clinical psychologist, nutritionist, rehabilitation specialist, occupational/physical therapist) based on the needs of the patient and his family. In the absence of the need for hospitalization, patients are seen on an outpatient basis at the Pain Clinic. Members of the pain management and palliative care team are accessible to patients 24/7 by phone. The management of the interdisciplinary team is guided by the pain management and palliative care guidelines established by the National Comprehensive Cancer Network (10).
The control arm consisted of standard pain management, pain diary that is accomplished once daily, and daily Pain Unit nurse call when pain score ≥7 until controlled (score ≤3). The intervention arm consisted of usual pain management and iComPAsS. Patients were followed up within 20±2 weeks from enrolment. Follow-up consisted of a clinic visit and questionnaires at baseline and at week 3, 6, 12 and 20.
The nature of the iComPAsS precludes blinding of the patient and physician. The research staff conducting the study assessments were different from those who conducted the randomization and were not allowed ask the patient about the iComPAsS.
Outcome measures
The Edmonton Symptom Assessment Scale (ESAS) is a simple, validated tool that was originally developed for screening and monitoring for the most common symptoms in patients with cancer. The ESAS uses a 0–10 numerical scale (0 is none, 10, worst) for symptoms including pain, fatigue, drowsiness, nausea, anxiety, depression, quality of appetite, dyspnea, sense of well-being, and one other symptom chosen by the patient (11). For our purposes, a pain score of 7–10 was severe, 4–6, moderate, 1–3, mild and 0, none (10). Controlled pain was defined as no or mild pain.
Compliance rate was defined as the percentage of patients who have adhered to the prescribed frequency of symptom-reporting, and was in four one-week tracking periods (week 3, 6, 12 and 20).
The Treatment Self-Regulation Questionnaire (TSRQ) was employed to measure the effect of the self-determination theory. The TSRQ consisted of a Perceived Competence Scale (PCS) for Pain Management, to gauge perceived capability for self-care; a Pain Management Questionnaire, to explore motivation behind compliance to pain medications and treatment; and a Program Participation Questionnaire, to explore motivation behind enrolling and continuing the symptom-reporting program. Responses are given using a 7-point Likert scale. Subscale scores are then derived to assess different forms of motivation: amotivation, external, introjection, identification and integration (12). The TSRQ was administered at baseline, week 6, 12 and 20.
Statistical analysis
Pain levels and compliance were compared between the groups using Student’s t-test. The Pearson Correlation Coefficient was used to examine the relationship between compliance to symptom-reporting and (I) pain control, (II) perceived competence in pain self-care, and (III) nature of motivation (intrinsic versus controlled-type) behind adherence to pain medication/treatment and entry to and continuation of participation in the symptom-reporting program. A correlation coefficient of 0.00–0.19 is considered very weak; 0.20–0.39, weak; 0.40–0.59, moderate; 0.60–0.79, strong, and 0.80–1.00, very strong (13).
Ethics statement
This study was reviewed and approved by the University of Santo Tomas Hospital—Institutional Review Board (IRB-MD-04-2015-054-A1).
Results
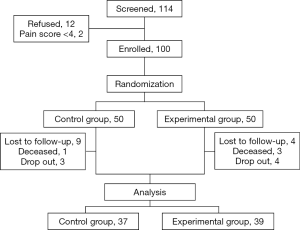
Out of 100 enrolled participants, 13 were lost to follow up, four died, and seven opted to drop out after controlled pain; 76 were included in this analysis—37 from the control arm and 39 from the experimental arm (Figure 2). The majority are female, married, with college degree, unemployed, with an annual income under USD 2,000, or living with family. There are no significant differences between the two arms in terms of demographic characteristics (Table 1).
Table 1
| Variables | Control group (N=37), n (%) | iComPAsS group (N=39), n (%) | P value |
|---|---|---|---|
| Age (mean ± SD) | 57.85±12.64 | 54.82±15.70 | 0.35 |
| Sex | 0.49 | ||
| Male | 17 (45.95) | 15 (38.46) | |
| Female | 20 (54.05) | 24 (51.54) | |
| Marital status | 0.45 | ||
| Single | 5 (13.51) | 7 (17.95) | |
| Married | 24 (64.86) | 24 (61.54) | |
| Common law partner* | 1 (2.70) | 1 (2.56) | |
| Widowed | 1 (2.70) | – | |
| Separated | 6 (16.22) | 7 (17.95) | |
| Educational attainment | 0.95 | ||
| Primary | 4 (10.81) | 4 (10.26) | |
| Secondary | 11 (29.73) | 9 (23.08) | |
| College | 20 (54.05) | 23 (58.97) | |
| Graduate | 1 (2.70) | 2 (5.13) | |
| Doctorate | 1 (2.70) | 1 (2.56) | |
| Religion | 0.67 | ||
| Catholic | 31 (83.78) | 32 (82.05) | |
| Non-Catholic | 6 (16.22) | 7 (17.95) | |
| Occupation | 0.34 | ||
| Employed | 9 (24.32) | 7 (17.95) | |
| Self-employed | 5 (13.51) | 1 (2.56) | |
| Housewife | 1 (2.70) | 3 (7.69) | |
| Unemployed | 17 (45.95) | 23 (58.97) | |
| Retired | 5 (13.5 | 5 (12.82) | |
| Annual Income | 0.52 | ||
| <USD 2,000 | 21 (56,76) | 26 (66.67) | |
| USD 2,000–4,000 | 4 (10.81) | 1 (2.56) | |
| USD 4,00–8,000 | 3 (8.11) | 3 (7.69) | |
| >USD 8,000 | – | 1 (2.56) | |
| No answer | 9 (24.32) | 8 (20.51) | |
| Living conditions | 0.59 | ||
| Alone | 1 (2.70) | 1 (2.56) | |
| With family | 32 (86.49) | 33 (84.62) | |
| With carer | 4 (10.81) | 4 (10.26) | |
| No answer | – | 1 (2.56) |
Baseline pain level (pain score 5.09) and self-regulating characteristics—perceived moderate competence for pain self-management, predominantly intrinsic motivation scores for adhering to pain medications/treatment and entering the symptom-reporting program [as indicated by moderately high Relative Autonomy Index (RAI) values]—were similar between the two groups (Table 2).
Table 2
| Clinical variable | Control (mean ± SD) | iComPAsS (mean ± SD) | P value |
|---|---|---|---|
| Pain level | 5.09±1.11 | 5.09±1.56 | 0.99 |
| Self-regulating characteristics | |||
| Perceived competence for pain self-management | 4.38±1.04 | 4.43±1.40 | 0.84 |
| Adherence to pain medications/treatment | |||
| Autonomous regulation | 5.49±1.28 | 5.77±1.22 | 0.32 |
| Controlled regulation | 4.82±1.19 | 4.95±1.57 | 0.69 |
| Relative Autonomy Index | 0.67±0.75 | 0.83±1.25 | 0.52 |
| Entry into the symptom-reporting program | |||
| Autonomous regulation | 5.59±1.27 | 6.04±1.77 | 0.10 |
| Controlled regulation | 4.41±1.34 | 4.51±1.77 | 0.78 |
| Relative Autonomy Index | 1.17±1.26 | 1.52±1.61 | 0.30 |
Overall compliance to symptom-reporting was significantly higher for the experimental arm (Table 3). At any point, compliance was higher for the experimental arm, and significantly so during weeks 3 and 12. Peak compliance rate was 64% in the experimental arm, observed at week 3 (first tracking period), compared to 38% in the control arm, at week 6 (second tracking period). Finally, low compliance (less than 20%) was observed in both arms at week 20. Percentage of patients with controlled pain was significantly higher (1.4 times) in the iComPAsS group compared to the control group at week 3.
Table 3
| Tracking period | Compliance | Pain Control | |||||
|---|---|---|---|---|---|---|---|
| Control, mean ±SD (%) | iComPAsS, mean ±SD (%) | P value | Control (%) | iComPAsS (%) | P value | ||
| Overall | 19.92±25.48 | 34.09±23.34 | <0.01 | ||||
| 3rd week | 26.12±35.56 | 64.29±28.04 | <0.01 | 49 | 69 | 0.02 | |
| 6th week | 37.71±50.27 | 48.81±31.30 | 0.24 | 71 | 68 | 0.70 | |
| 12th week | 19.11±32.67 | 36.88±32.06 | 0.02 | 72 | 69 | 0.83 | |
| 20th week | 11.61±31.02 | 15.57±24.96 | 0.54 | 78 | 65 | 0.43 | |
For other symptoms, including anxiety and depression, severity levels at baseline and throughout the course of the study were mild and generally stable (Table 4).
Table 4
| Symptom | Baseline | 3rd week | 6th week | 12th week | 20th week | P value |
|---|---|---|---|---|---|---|
| Control | ||||||
| Pain | 5.09±1.11 | 3.72 +2.60 | 2.62±3.10 | 2.32±2.79 | 2.00±2.88 | <0.01 |
| Tiredness | 2.44±2.47 | 1.74±2.04 | 1.22±1.98 | 0.75±1.46 | 1.43±1.99 | <0.01 |
| Drowsiness | 2.05±2.33 | 1.62±2.03 | 1.32±2.17 | 1.25±1.96 | 1.29±1.97 | 0.20 |
| Nausea | 1.02±2.15 | 0.79+1.79 | 0.22+0.89 | 0.14±0.76 | 0.14±0.53 | 0.05 |
| Lack of appetite | 2.23±2.94 | 1.95±2.47 | 1.03±2.03 | 1.50±2.44 | 0.71±1.27 | 0.05 |
| SOB | 0.95±2.02 | 0.79±1.59 | 0.55±1.19 | 0.64+1.22 | 1.14±1.79 | 0.60 |
| Depression | 1.58±2.18 | 1.03 +1.75 | 0.51±1.33 | 0.75±1.51 | 0.79±2.15 | 0.01 |
| Anxiety | 1.60±2.40 | 0.72±1.89 | 0.62±1.57 | 0.75±1.46 | 0.71±1.90 | 0.41 |
| Poor well-being | 3.53±2.02 | 2.87±1.82 | 2.14±1.87 | 2.46±2.27 | 2.21±1.48 | <0.01 |
In the iComPAsS group, compliance positively correlated, although weakly, with uncontrolled pain (0.33) and an intrinsic motivation for entry into the program (0.25) and for continued program participation (0.28). None of the factors significantly correlated with compliance in the control group (Table 5).
Table 5
| Factor | Association with compliance (Pearson correlation coefficient) | |
|---|---|---|
| Control | iComPAsS | |
| Uncontrolled pain | 0.13 | 0.33 |
| Perceived pain management competence | −0.17 | −0.20 |
Discussion
Self-determination theory is an effective theoretical basis for various intervention and prevention programs. This analysis examined how the theory applies to a mobile application designed to permit remote symptom-reporting towards optimized pain and symptom monitoring and management and eventually, improved health-related outcomes.
In both groups, patients had high RAI values (intrinsic motivation) (Table 2) and the manner of introduction of the pain diary and the mobile application were similar, and included a detailed orientation on the rationale (thus addressing the autonomy element) and the use of the tools (thus addressing the competence element). However, compliance during the first tracking period (week 3) was significantly (2.5 times) greater for the iComPAsS group than for the control group (Table 3), reflecting a quicker uptake of the mobile application. While this may partly relate to our inclusion criteria (mobile phone access and willingness to learn and use a telemedicine application), the incorporation of a video tutorial and a Help function in the application, likely enhances adoption of the technology by further addressing the competence element.
The higher uptake rate in the experimental arm was associated with a significantly higher percentage of patients with controlled pain (49% versus 69%, in favour of iComPAsS, P=0.02) and thus a shorter time to pain control. This affirms the clinical utility of a remote symptom-reporting tool, facilitating more efficient pain management.
The subsequent tracking period showed an upward trend (+44%) for compliance in the control arm, and the reverse (−24%) for the iComPAsS, reflecting the effect of pain control on compliance. During this period, there was an associated increase in the percentage of patients with controlled pain in the control group, and a stable trend in the other. The third tracking period saw a significant drop (−50%) in compliance in the control group, suggesting compliance driven largely by lack of pain control than intrinsic motivation. The drop is less significant (−24%) for the iComPAsS arm, suggesting sustained compliance that is driven by intrinsic motivation. Thus, in the iComPAsS arm, uncontrolled pain and sustained intrinsic motivation both correlate with continuing compliance to the program. This sustained intrinsic motivation may be attributed to the provision of real-time Notifications in the iComPAsS, which addresses the relatedness element more effectively than the daily nurse calls in the control group.
By better incorporating features that address all three elements of the self-determination theory, the iComPAsS was able to elicit a more rapid initial uptake, to achieve the desired clinical effect more promptly, and to effectuate a more sustained compliance, when compared to the control group. If it is to be introduced as part of standard practice, these features need to be identified, maintained and reinforced. The following recommendations for each element are two-fold, and directed to the application and to the healthcare professional.
Autonomy-supportive elements, which create a conducive environment that would assure the patient that he is understood, regarded positively and unconditionally, and permitted to work at his own pace, are key (14). The patient must be presented a clear rationale for the recommended behavior (symptom reporting), a clear mechanism of the process (how symptom reporting might lead to a more efficient symptom management and improved health outcomes), and an assurance that he is regarded as an important player in the process (15,16). These will foster a sense of ownership, commitment and active involvement (17). While these have been addressed in the conception and design of the iComPAsS (by providing a video tutorial and a Help section), presentation of the application as a tool rather than a task was as important and should be done separately from the tutorial for its use. Framing that the mobile application is a device that listens, offers alternatives and make things easier and more practical for the patient addresses the autonomy element of motivation. This will ensure continued use of the application.
For the competence element, it is recommended that informational resources on skills and knowledge on symptom management, supportive tools for behavioral implementation, and feedback mechanism in the forms of reminders and brief reports for the patient’s progress be included in the final version of the application (18). The healthcare professional should provide relevant information about the patient’s current condition, symptoms, treatment, and prognosis in a timely manner. This will foster in the a patient a sense of being in control and reduce anxiety. Autonomous disengagement entails allowing the patient for an educated decision on whether to report their symptoms or not and to choose a medium that is relevant to them at a given moment. Thus, they should be given access to all available platforms, such as the traditional face-to-face and electronic mobile format. The advantages and disadvantages of one over the other must be discussed collaboratively with the patient. Thus, the patient may anticipate or report any barriers in the use of the mobile application and, together with the healthcare professional, find potential ways to address them.
For the relatedness element, social support within the context of cyberspace such as provision of web blogs, bulletin board systems or just an easy access to community online or the healthcare professional may be included (18). A strong feedback mechanism must be put in place that will reinforce symptom-reporting behavior, address negative comments about the application or symptom management in general. Timely response from the healthcare professional is key and combining personal and electronic approaches during the first sessions with the patient might be valuable. Patients prefer a stronger therapeutic relationship with their healthcare professionals so introducing them to mobile application and completely removing the personal meetings will not be very effective. Feedback must also be provided in a non-controlling language, with provision of choices and coupled with verbal explanations to help them understand the process better. In this manner of presentation, the patients would participate more actively.
On the other hand, it must be noted that our cohort had moderately high baseline levels of intrinsic motivation and mild levels of accompanying symptoms, which remained generally stable throughout the follow-up. In general, drop-out rates increase through time in several studies that employ technology as a platform compared to face-to-face meetings. When implementing this in clinical practice, it might be useful to use the TSRQ to gauge the baseline levels of motivation of the patient, and to screen for other factors that might hamper engagement, such as depressive symptoms, which are common among cancer patients especially those with severe or debilitating pain (19). These information could direct the healthcare practitioner to employ adjunct measures that can enhance uptake and sustained compliance, or an alternative, better suited approach.
Finally, compliance was largely influenced by uncontrolled pain, and despite a relatively sustained compliance consistent with intrinsic motivation in the iComPAsS group, it must be noted that after about three months of use, compliance has consistently declined despite lack of further increase in pain control. This might suggest that for the patient, the utility of this tool is limited to the early stages of pain management (initiation and dose titration) rather than for longer-term pain and symptom monitoring. Currently, an enhanced version of the app is being developed to cater to cancer patients with acute pain and patients with pain in the perioperative period.
Conclusions
By successfully addressing all three elements of the self-determination theory, the iComPAsS program is able to elicit a more rapid uptake, to achieve the desired clinical effect promptly, and to effectuate sustained compliance through intrinsic motivation. Uncontrolled pain and sustained intrinsic motivation correlate with continuing compliance to the program.
When adopting the iComPAsS for clinical use, the TSRQ may be employed to gauge baseline motivation levels, and patients must be screened for barriers to engagement, such as depressive symptoms, which are common among cancer patients. These information could direct the healthcare practitioner to employ adjunct measures that can enhance uptake and sustained compliance to the program, or an alternative, better suited approach.
Acknowledgments
Funding: This project was supported by the ASCO Conquer Cancer Foundation Grant.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jhmhp.2018.04.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the University of Santo Tomas Hospital—Institutional Review Board (IRB-MD-04-2015-054-A1). A written informed consent form was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol 2000;55:68-78. [Crossref] [PubMed]
- Roca JC, Gagné M. Understanding e-learning continuance intention in the workplace: A self-determination theory perspective. Computers in Human Behavior 2008;24:1585-604. [Crossref]
- Patrick H, Williams GC. Self-determination theory: its application to health behavior and complementarity with motivational interviewing. Int J Behav Nutr Phys Act 2012;9:18. [Crossref] [PubMed]
- Orsini C, Evans P, Jerez O. How to encourage intrinsic motivation in the clinical teaching environment?: a systematic review from the self-determination theory. J Educ Eval Health Prof 2015;12:8-10. [Crossref] [PubMed]
- Quartiroli A, Maeda H. Self-determined engagement in physical activity and sedentary behaviors of us college students. Int J Exerc Sci 2014;7:87-97. [PubMed]
- Ng JYY, Ntoumanis N, Thøgersen-ntoumani C, et al. Self-determination theory applied to health contexts: a meta-analysis. Perspect Psychol Sci 2012;7:325. [Crossref] [PubMed]
- Coa K, Patrick H. Baseline motivation type as a predictor of dropout in a healthy eating text messaging program. JMIR Mhealth Uhealth 2016;4:e114 [Crossref] [PubMed]
- Hull SJ, Abril EP, Shah DV, et al. Self-determination theory and computer-mediated support: modeling effects on breast cancer patient’s quality-of-life. Health Commun 2016;31:1205-14. [Crossref] [PubMed]
- Choi J, Kushner KE, Mill J, et al. Understanding the language, the culture, the experience: translation in cross cultural research. Int J Qual Methods 2012;11:652-65. [Crossref]
- National Comprehensive Cancer Network. Adult cancer pain. NCCN Clinical Practice Guidelines in Oncology Version 1.2018. Accessed 24 January 2018.
- Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6-9. [PubMed]
- Levesque CS, Williams GC, Elliot D, et al. Validating the theoretical structure of the Treatment Self-Regulation Questionnaire (TSRQ) across three different health behaviors. Health Educ Res 2007;22:691-702. [Crossref] [PubMed]
- Evans JD. Straightforward Statistics for the Behavioral Sciences. Pacific Grove, Calif.: Brooks/Cole Publishing, 1996.
- Reeve J. Why teachers adopt a controlling motivating style toward students and how they can become more autonomy supportive. Educational Psychologist 2009;44:159-75. [Crossref]
- Williams GC, McGregor HA, Sharp D, et al. Testing a self-determination theory intervention for motivating tobacco cessation: Supporting autonomy and competence in a clinical trial. Health Psychol 2006;25:91-101. [Crossref] [PubMed]
- Mageau GA, Vallerand RJ. The coach-athlete relationship: A motivational model. J Sports Sci 2003;21:883-904. [Crossref] [PubMed]
- Ryan RM, Deci EL. A self-determination theory approach to psychotherapy: the motivational basis for effective change. Can Psychol 2008;49:186-93. [Crossref]
- Choi J, Noh GY, Park DJ. Smoking cessation apps for smartphones: content analysis with the self-determination theory. J Med Internet Res 2014;16:e44 [Crossref] [PubMed]
- Que JC, Sy Ortin TT, Anderson KO, et al. Depressive symptoms among cancer patients in a Philippine tertiary hospital: prevalence, factors, and influence on health-related quality of life. J Palliat Med 2013;16:1280-4. [Crossref] [PubMed]
Cite this article as: Bacorro WR, Balid-Attwell SA, Sogono PG, Escuadra CJT, Reyes-Gibby C, Que JC, Sy Ortin TT. Factors in sustained compliance to a symptom-reporting mobile application: implications for clinical implementation. J Hosp Manag Health Policy 2018;2:19.